Which of the Following Best Describes an Atom
Protons neutrons and electrons all grouped together in the center. 14 How do we.
Which statement best describes an atom.

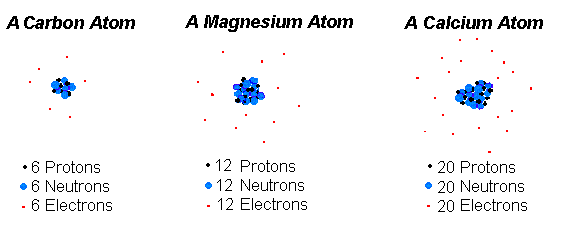
. 9 Which statement best describes the part of the atom that is shown by the arrow. One carbon atom forms a double bond with an oxygen atom and two single bonds with two hydrogen atoms. The number of neutrons in the atom the number of protons electrons and neutrons in the atom the number of protons in the atom the number of electrons in the atom Question 17 The sodium atom contains 11 electrons 11 protons and 12 neutrons.
An atom is neutral. Protons jump to higher energy levels and specific wavelengths are emitted as the protons return to the ground state. Which of the following best describes the atomic number of an atom.
C Electronegativity is the energy lost when an atom gains an electron. Apositive nucleus surrounded by a cloud of negative charges best describes an atomic structure. B It would form ions with a -2 charge.
Question 18 Which of the following best describes the atomic number of an atom. 16_ 16 Which of the following best describes how an atom with atomic number 12 would behave in terms of forming bonds with other elements. A Structurally variant atoms which always have a mass number of 1.
What is the charge of the atoms nucleus. 20 Questions Show answers. An atom consists of a central nucleus with proton neutrons and electrons orbiting in levels of high probability.
Protons and electrons grouped together in a random pattern. 2 on a question. A core of electrons and neutrons surrounded by.
Atom is the basic unit of matter. 10 How are electrons arranged in an atom. 11 Which of the following statements correctly defines the atomic mass of an element.
A core of electrons and neutrons surrounded by protons. Which of the following best describes a possible carbon compound. B Electronegativity is the attraction an elements nucleus has for the electrons in a chemical bond.
O A core of negatively and positively charged particles surrounded by neutral particles. A Electronegativity is what happens when an atom gains an electron to become an anion. Due to this high probability orbits are replaced by the term orbital.
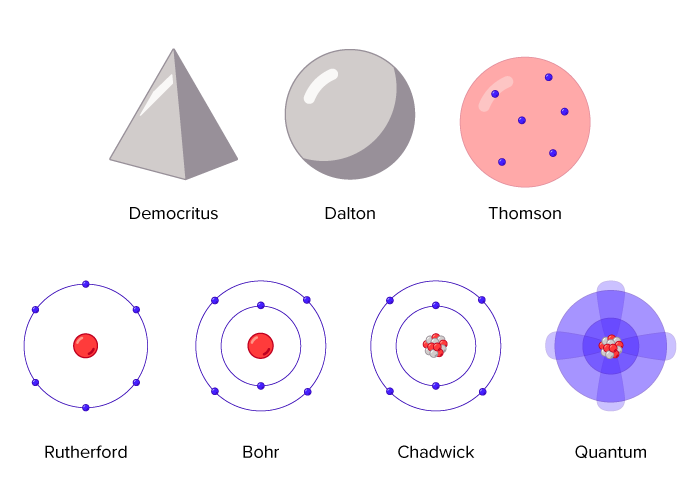
Core of positive and neutral particles surrounded by negative particles. According to the Bohrs atomic model the electrons revolve around the nucleus in a fixed orbit. A core of electrons and neutrons surrounded by protons.
Protons and electrons grouped together in an alternating pattern. B Structurally variant atoms which have the same number of neutrons and protons but differ in the number of electrons they contain. One carbon atom forms five single bonds with five hydrogen atoms.
Which of the following best describes an atom. A the number of protons in the atom B the number of electrons in the atom C the number of neutrons in the atom D the number of protons electrons and neutrons in the atom E the net electrical charge of the atom. From the given choices the best answer would be.
85A Which of the following best describes an electron. 2Which subatomic particles is electrically neutral. What is an atom.
Core of positive and neutral particles surrounded by negative particles. View the full answer. Proton 3Which among the following.
Protons and neutrons are located in the cloud around the nucleus and electrons are located in the nucleus of the atom. Tha atom is consisting of the following parts. 85A Which of these best describes one of the subatomic particles that could be found at location X in the model of an atom shown above.
One carbon atom forms a quadruple bond with another carbon atom. Protons neutrons and electrons all grouped together in the center. Which of the following best describes an atom.
See answers 2 Best Answer. The current model of an atom is best described by the Solar System. 85A An atom has 25 protons 30 neutrons and 25 electrons.
It would form ions with a 2 charge. Which of the following statement best describes an 1Which of the following statement best describes an atom. An atom is the basic unit of all things in the universe.
A It would form two covalent bonds with other atoms. A core of protons and neutrons surrounded by electrons. Which of the following best describes an isotope.
The planets represent electrons while the sun represents the nucleus. Which of the following explanations BEST describes how an atom emits color. Atom is made up of.
Protons jump to higher energy levels and specific wavelengths are emitted as the protons move to the excited. According to the new current Quantum atomic model An orbital is a region of space in an atom where the. Which one of the following statements best describes electronegativity in atoms.
Which of the following best describes an atom. 13 What atomic model accurately explains the structure of an atom. Which of the following best explains what is happening when an atom emits light.
Protons and electrons are located in the cloud around the nucleus and neutrons are located in the nucleus of the atom. D It would form ions with a 1 charge. O A core of negatively and positively charged particles surrounded by neutral particles.
E It would form ions with a -1 charge. 12 How are the elements arranged in the periodic table. It is the tiniest particle of an object.
One carbon atom forms a triple bond with another. What phase best describes an atom. An electron is dropping from a higher to a lower energy level with the difference in energy between the two being emitted as light energy.
Atomic number also called proton number refers to the.

Atom Test 8th Grade Science Multiple Choice Test Atom
0 Response to "Which of the Following Best Describes an Atom"
Post a Comment